

Achieving P4 Goals Using Ozone Reaction Chemistry and
Dynamic Surface Tension Measurement in a New Process
Control Scheme to Form Steady State Cleaning Processes
In the Beginning – Let there be Waste!
Early pollution control technologies and methods focused primarily on end of pipe treatment and disposal. Improper waste disposal, mismanagement, increased legal liability, toxic torts, fewer disposal sites, escalating disposal and treatment costs, stricter treatment requirements, and outlawed processes and chemistries (Montreal Protocol and the Kyoto Protocol) have all contributed to the need for a new direction in waste management control. This new direction has come to be called P4 (Permanent Pollution Prevention Planning).
Shifting Economics – Thou shalt not Waste Resources!
Recognizing the increasingly negative effect on bottom line profits, and faced with replacing outlawed cleaning methods and chemistry, industry recently began to look for more cost effective ways to reduce environmental compliance costs. The golden age of seemingly unlimited resources during the early days of the industrial revolution had come to an abrupt end. Originally the primary focus was on minimizing labor and capital production costs. This led to manufacturing processes that produced huge volumes of toxic and hazardous waste materials and resulted in the inefficient use of many raw materials which were cheap and plentiful at the time. The result of these events has been a massive shift in manufacturing process economics. The new focus is zero reject, zero waste, and zero discharge manufacturing. The goal is 100 % conversion of raw materials into saleable products.
What is P4 and how did we get here?
In 1990 Congress passed new legislation mandating the creation and implementation of Pollution Prevention Plans ( November 5, 1990, Pollution Prevention Act of 1990). Federal, state, and local governments, industrial facilities that generate hazardous waste (Small Quantity and Large Quantity Generators) and TRI (Toxic Release Inventory) reporters are all required to create and implement Permanent Pollution Prevention Plans.
P4 Goals
The goal of the P4 process is to reduce raw materials consumption, control pollution and liability costs, protect the environment and reduce risks to worker health and safety. New emphasis is also placed on multi media pollution control to stop conversion of one pollutant into another. Examples of Multi-Media pollution Transport include:
- Waste water sludge produced to clean up waste water moves contaminates from a water body to a land fill.
- Sludge burning creates air contaminates in the process of destroying the (solid) sludge.
- Dust collectors move the air pollution to a landfill.
The first step in P4 implementation is source reduction. Source reduction is achieved primarily by modifying the production process to minimize waste production at the source. This reduces the quantity and or toxicity of any waste that is produced. The second step is to find ways to recycle any wastes that are produced. Redesigning processes to produce a minimal amount of a recyclable waste is part of this process.
P4 Analysis - New Needs - New opportunities - New Economics
Recent advancements in semiconductor electronics, sensors, process miniaturization, control, reduced data acquisition costs and increased capabilities have created many new opportunities. Combined with the new P4 goals and economic realities many previously inconceivable schemes become practical. By combining several new technologies and novel approaches to P4 implementation the promise of zero discharge finally becomes conceivable.
Traditional solutions to P4 implementation have included the use of standard chemical engineering physical separation process methods. These methods include mechanical filtration, skimmers, micro-filtration, ultra-filtration, reverse osmosis, de-ionization, distillation and forced evaporation. These methods can extend cleaning bath life but typically have very high capital and or operating cost penalties. They frequently consume large amounts of additional energy and / or chemicals to accomplish their goals. Finally they usually produce one or more additional waste streams adding to the waste management burden.
A new approach is therefore needed. One of the fundamental principles in the world of Martial Arts is to use a greater force against itself. One implementation of this principle in the world of parts cleaning is to find a way to convert cleaning contaminates (the greater force) into cleaning agents. Large quantities of organic compounds (crude oil derivatives, animal fats, etc.) are converted into cleaning surfactants at major chemical manufacturing plants throughout the world. These same organic compounds (oils and greases), which are used as feed stocks to manufacturer surfactants, are also used as lubricants and cutting fluids. They eventually require cleaning and removal from parts being cleaned prior to further processing. At this point they become the cleaning solution contaminates (the greater force).
By bringing the chemical manufacturing process to the point of use (the cleaning process) we convert the contaminate (using the greater force against itself), as it accumulates in the cleaner bath, into useful cleaning agents (surfactants, emulsifiers, etc.). This eliminates most onsite and offsite storage and transport hazards, reduces risk to worker safety and health, protects the environment, reduces raw materials consumption and eliminates pollution and liability costs.
Step One – Conversion of Organic Oily Contaminates into Surfactants Onsite
The basic chemistry of surfactants and their manufacture is well documented in prior articles and is not the focus of this article. In general cleaning surfactants contain a hydrophobic (water hating and usually contain hydrocarbon chains) end and a hydrophilic end (water loving). The hydrophilic end usually contains oxygen, and sometimes nitrogen, fluorine, or other electrophilic species. Mild oxidation of oily contaminates with oxygen containing compounds, and free radical reaction with oxygen containing free radicals produces surfactants containing hydrophilic functional groups such as alcohols, esters, carbonyl, and carboxylic acids attached to hydrophobic hydrocarbon chains.
Ozone reacts directly with organics by attacking unsaturated carbon to carbon double bonds. Some reaction products of ozonation include organic peroxides, hydrogen peroxide, and superoxide anion. These organic peroxides can react non-selectively with other saturated or unsaturated compounds in the recycled cleaner through a free radical reaction mechanism, thus forming new compounds through a coupling process that is similar to polymerization. This results in the creation of a wide variety of surface active compounds in the recycled cleaner/ozone process.
After repeated exposure of recycled cleaning solutions to limited amounts of ozone, new surfactants begin forming that are amphoteric (soluble in acids and bases) with alcohol, glycol, and carboxylic acid functional groups, all on the backbone of the hydrocarbon.
These surfactants variably exhibit high solubilities in water, the ability to chelate and sequester metal ions, and can increase the cleaner's ability and capacity to emulsify and solubilize hydrophobic contaminants. Fig. 1 shows typical dynamic surface tension values of a used alkaline cleaning solution before and after treatment in the reactor with ozone.
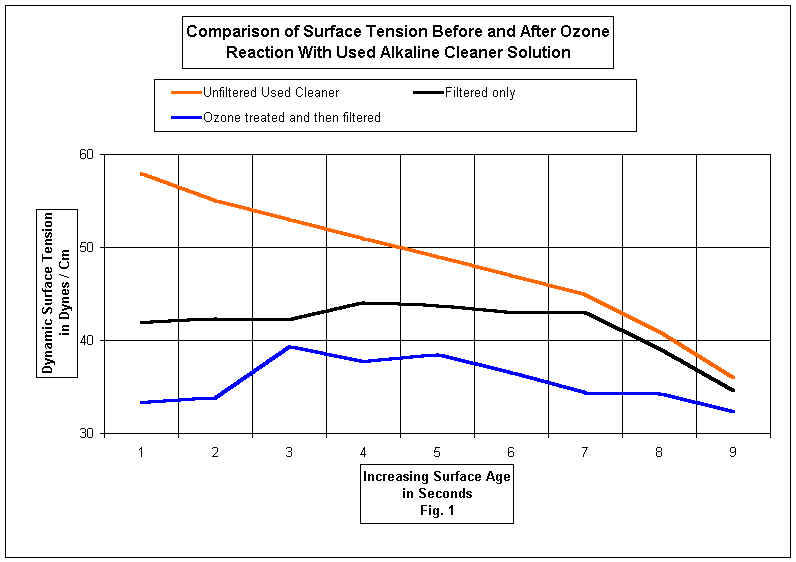
Either a batch reactor or a continuous on line reactor system can be installed on existing parts washer systems. Current systems use a novel reactor design that utilizes ozone as a limiting reagent in the reactor to convert oily contaminates into useful surfactants. In some cases cleaner performance is not only restored but enhanced by converting a short lived low cost cleaner into a more effective long life emulsion cleaner.
This process is described in much greater detail in another article I wrote in the September 2000 issue of Parts Cleaning Magazine which can be found on the CleanTech Magazine Web Site.
Process Control
Batch reactors can be operated on a manual empirical basis. Periodic samples can be pulled and tested using several lab procedures such as alkalinity titration, pH, oil and grease, emulsifying or solublizing capacity, cleaning standard test panels, etc. Based on historical performance and test data, decisions can be made as to when to run the reactor and when to turn it off. This is similar to pulling grab samples of a used cleaning bath, testing it and then deciding based on the test data how much raw cleaner additive to add back into the bath to restore the bath’s performance.
Many parts washer processes already have temperature and or liquid level controls built into the systems. Systems to measure and control pH, pump or nozzle pressure are also available. The prior presentation today covered another parameter that can be measured. This parameter is surface tension. Static surface tension measurement of used cleaning solutions offers very little assistance due to phase splitting and resulting interferences. Direct measurement of active cleaning component and contaminate concentrations is complex, costly and impractical in most cases. Dynamic surface tension (prior paper presentation) is a function of temperature, pH, and the concentration of all active compounds (cleaning agents and contaminates) in the cleaning solution. No other single test parameter can yield as much useful data in one step as a dynamic surface tension measurement can yield for every day liquid cleaning processes.
The dynamic surface tension of cleaning solutions can be monitored by periodically pulling and testing grab samples. Critical upper and lower dynamic surface tension values (or slopes) can then be determined based on real part cleaning performance (such as coating adhesion tests for parts that are painted after cleaning). This can then be used as the basis for reactor process on / off operation and control. Reactor operation can then be controlled on a manual basis (on / off) based on grab sample test results.
The disadvantage of batch processing is that cleaning cycle times are controlled by the dirty cleaning solutions capacity before dumping or regenerating. By adding continuous process control and treatment to existing cleaning systems we can reduce cleaning cycle times and / or operating temperatures. By increasing the flow through capacity (rate) of existing and new capital equipment it is possible to substantially reduce per part production costs. Fig. 2 shows a graphic representation and comparison of process efficiency for batch operations versus steady state continuous optimized systems. As you can see from the graph batch systems are operated at a rate based on insuring that parts are still cleaned just before a bath dump. It is easy to see from this graph that increased flow through rates of several hundred percent become possible when cleaning capacity is maintained at the optimal level on a continuous basis.
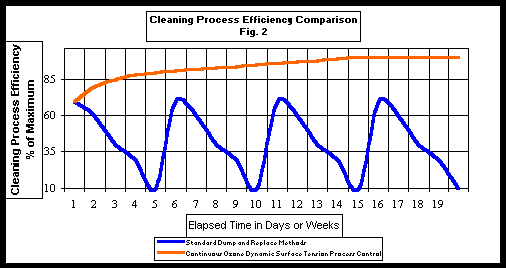
Continuous process control lowers overall energy costs and allows users to maintain steady-state cleaning line speeds. By maintaining continuous optimal cleaning chemistry conditions, cleaning costs are minimized. Under these conditions cleaning processes would no longer be dependent on frequent bath dumps to get line speeds back up or to reduce washer operating temperatures. This simultaneously reduces the need for energy and chemical consuming waste treatment cleanup steps and their capital and maintenance costs.
In addition to the basic reactor, in-process control sensors can be added to determine the cleaner’s dynamic surface tension, temperature, pH, turbidity and Total Organic Carbon content. These parameters can be set as control points for process control which can then regulate the cleaners critical properties by varying the ozone feed rate, pH, reactor temperature, make up additive addition, and the bleed rate to a bioreactor conversion stage to remove accumulated excess organics and salts. The overall efficiency of the process can be further increased by a synergistic combination with any currently available bioreactor technology. Using bioreactors to convert excess soap (surfactants) and emulsified oil into fertilizer (biomass) can result in a zero-discharge process.
Conventional activated-sludge municipal wastewater treatment systems and small industrial systems can readily biodegrade all ozone reaction products. The bioreactor stage ensures that the cleaning process does not eventually turn into a bar of soap. Facilities with existing biological waste water treatment systems could discharge a small flow (bleed) to their existing waste water treatment plants. The entire process can actually result in a positive (net gain) energy balance if used to increase or maintain line speeds at optimum levels, thereby increasing net production of clean parts per hour, or if used to lower operating temperatures at the same time.
An additional benefit is improved QA/QC (Quality Assurance / Quality Control). Highly critical cleaning processes can benefit further from the higher quality "continuous" data produced. There is far less chance of finding several hours or days of defective production work that could be of questionable quality if cleaning chemistry data is produced continuously and immediately at the point of use as opposed to hours or days later with batch grab sample testing. Frequently QA/QC product quality concerns force users to dump cleaning baths prematurely. This can substantially increase waste production volumes and waste treatment costs.
Summary
By using on site surfactant manufacturing technology, dynamic surface tension measurement, temperature measurement, pH measurement and control, liquid level or net volume control (bath volumes and liquid levels vary with temperature) and in some cases turbidity and conductivity measurement, a process control scheme can be developed that is specific to the individual user’s cleaning process needs. Integrated systems such as these will become the ultimate Permanent Pollution Prevention systems. They will decrease waste volumes to near zero levels, decrease operating costs, reduce energy consumption, chemical purchases, and reduce waste treatment and capital costs. Final product quality and quality control will benefit. Fewer rejects (another source of waste and pollution) will be produced. Benefits from increased productivity will be achieved by those who retrofit existing systems. With the continued tightening of environmental regulations many will find these options to be extremely attractive.
About the Author
Mike McGinness is Vice President of Technology at EcoShield Environmental Systems and holds a degree in Chemical Engineering from the University of Houston. He has 26 years of experience in industrial metal finishing processes and chemistry.
EcoShield Environmental Systems, Inc. · P.O. Box 1476 · Houston TX, 77251 · USAToll Free (877) 326-7443 · Fax (877) 326-9090 · www.ecoshieldenv.com